Highlighted publications
Frequency Selective Neuronal Modulation Triggers Spreading Depolarizations in the Rat Endothelin-1 Model of Stroke
Paolo Bazzigaluppi, James Mester, Illsung L Joo, Iliya Weisspapir, Adrienne Dorr, Margaret M Koletar, Tina L Beckett, Hourman Khosravani, Peter Carlen, and Bojana Stefanovic
Journal of Cerebral Blood Flow & Metabolism. 2021 Oct;41(10):2756-2768. Download the full text article here.
Abstract - Ischemia is one of the most common causes of acquired brain injury. Central to its noxious sequelae are spreading depolarizations (SDs), waves of persistent depolarizations which start at the location of the flow obstruction and expand outwards leading to excitotoxic damage. The majority of acute stage of stroke studies to date have focused on the phenomenology of SDs and their association with brain damage. In the current work, we investigated the role of peri-injection zone pyramidal neurons in triggering SDs by optogenetic stimulation in an endothelin-1 rat model of focal ischemia. Our concurrent two photon fluorescence microscopy data and local field potential recordings indicated that a ≥ 60% drop in cortical arteriolar red blood cell velocity was associated with SDs at the ET-1 injection site. SDs were also observed in the peri-injection zone, which subsequently exhibited elevated neuronal activity in the low-frequency bands. Critically, SDs were triggered by low- but not high-frequency optogenetic stimulation of peri-injection zone pyramidal neurons. Our findings depict a complex etiology of SDs post focal ischemia and reveal that effects of neuronal modulation exhibit spectral and spatial selectivity.
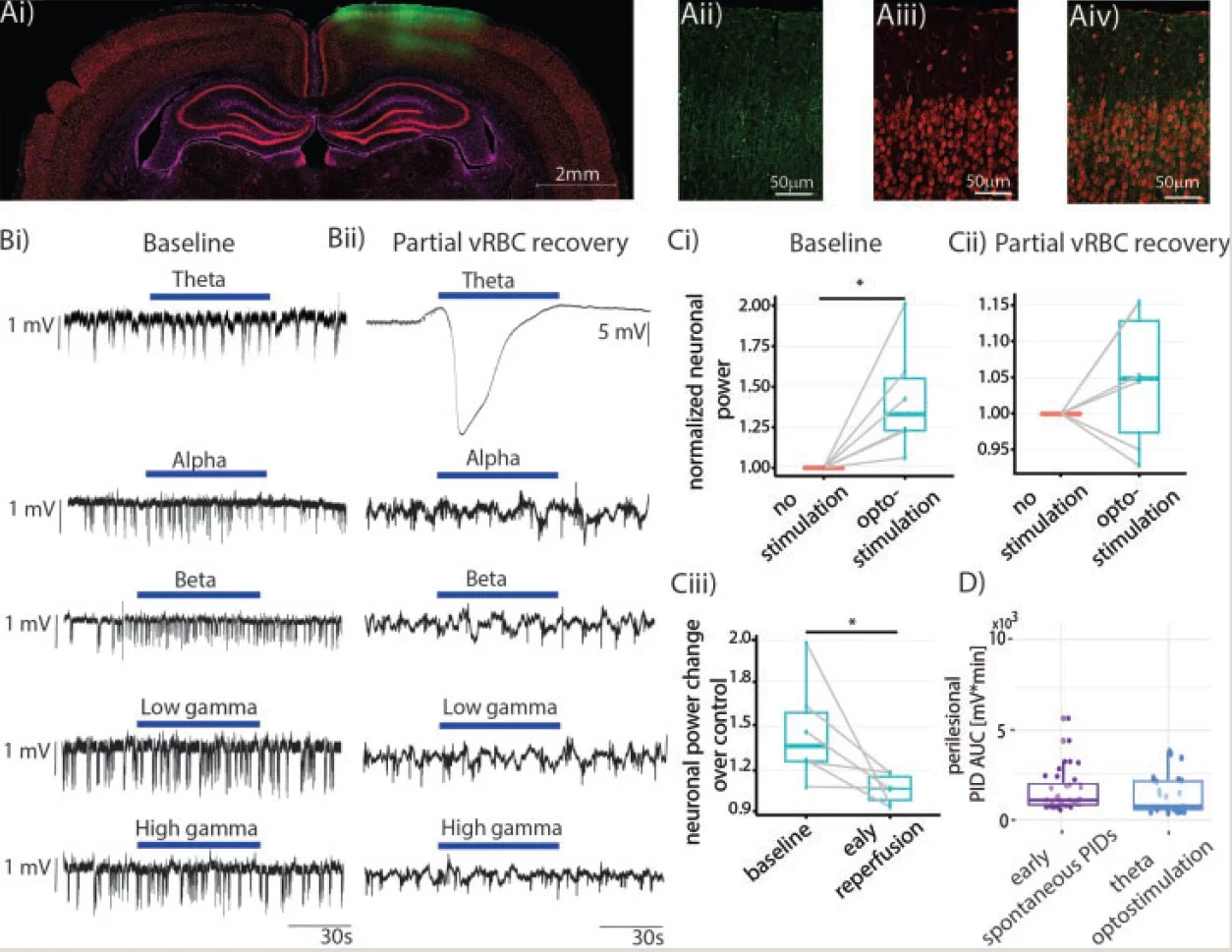
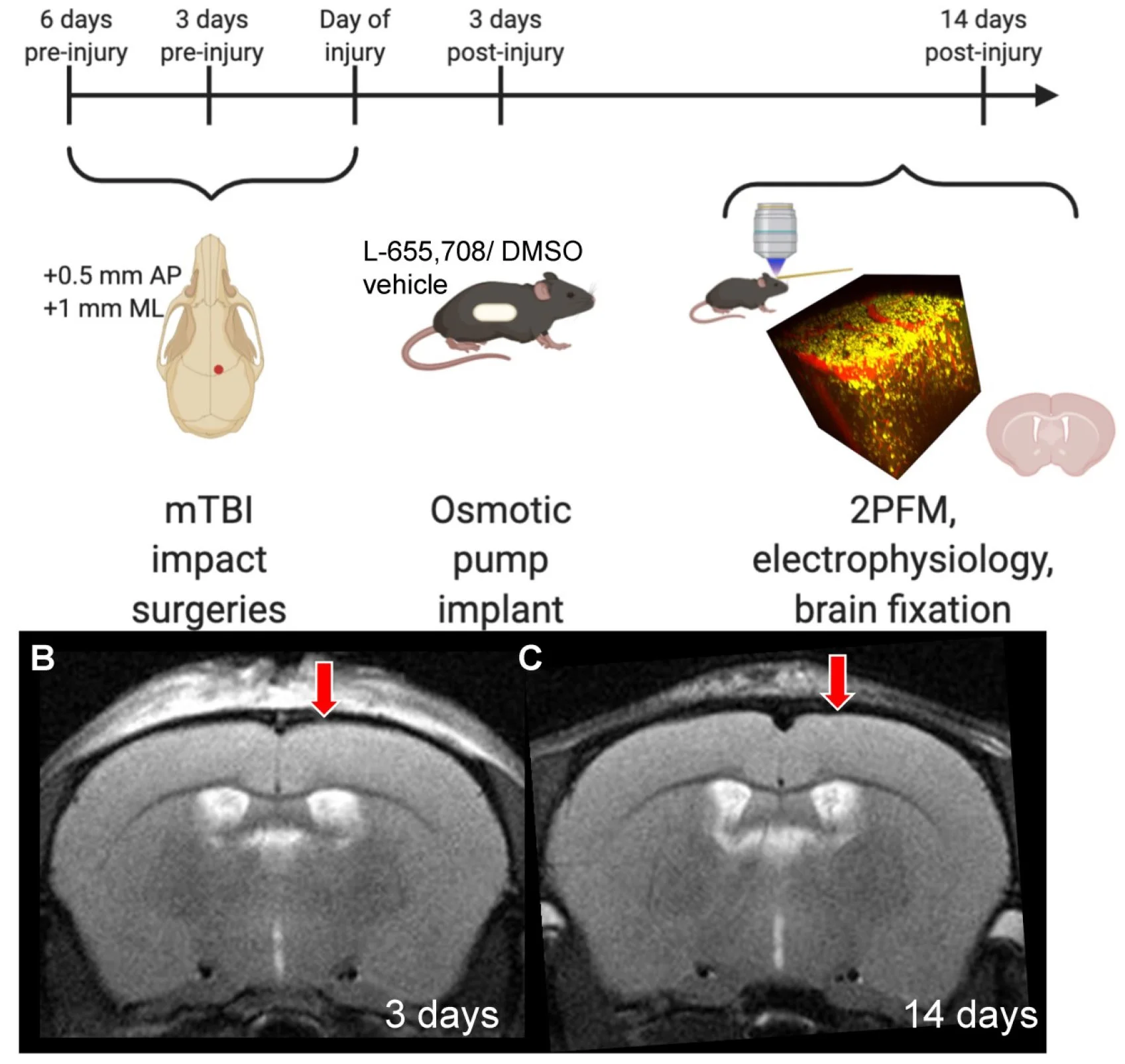
Attenuation of Tonic Inhibition Prevents Chronic Neurovascular Impairments in a Thy1-ChR2 Mouse Model of Repeated, Mild Traumatic Brain Injury
James R Mester, Paolo Bazzigaluppi, Adrienne Dorr, Tina Beckett, Matthew Burke, JoAnne McLaurin, John G Sled, Bojana Stefanovic
Theranostics. 2021 Jun 16;11(16):7685-7699. Download the full text article here.
Abstract - Rationale: Mild traumatic brain injury (mTBI), the most common type of brain trauma, frequently leads to chronic cognitive and neurobehavioral deficits. Intervening effectively is impeded by our poor understanding of its pathophysiological sequelae. Methods: To elucidate the long-term neurovascular sequelae of mTBI, we combined optogenetics, two-photon fluorescence microscopy, and intracortical electrophysiological recordings in mice to selectively stimulate peri-contusional neurons weeks following repeated closed-head injury and probe individual vessel's function and local neuronal reactivity. Results: Compared to sham-operated animals, mTBI mice showed doubled cortical venular speeds (115 ± 25%) and strongly elevated cortical venular reactivity (53 ± 17%). Concomitantly, the pericontusional neurons exhibited attenuated spontaneous activity (-57 ± 79%) and decreased reactivity (-47 ± 28%). Post-mortem immunofluorescence revealed signs of peri-contusional senescence and DNA damage, in the absence of neuronal loss or gliosis. Alteration of neuronal and vascular functioning was largely prevented by chronic, low dose, systemic administration of a GABA-A receptor inverse agonist (L-655,708), commencing 3 days following the third impact. Conclusions: Our findings indicate that repeated mTBI leads to dramatic changes in the neurovascular unit function and that attenuation of tonic inhibition can prevent these alterations. The sustained disruption of the neurovascular function may underlie the concussed brain's long-term susceptibility to injury, and calls for development of better functional assays as well as of neurovascularly targeted interventions.
Combinatorial Treatment Using Umbilical Cord Perivascular Cells and Aβ Clearance Rescues Vascular Function Following Transient Hypertension in a Rat Model of Alzheimer Disease
Paolo Bazzigaluppi *, Tina L Beckett *, Margaret M Koletar, Mary E Hill, Aaron Lai, Arunachala Trivedi, Lynsie Thomason, Adrienne Dorr, Denis Gallagher, Clifford L Librach, Illsung L Joo, JoAnne McLaurin, & Bojana Stefanovic (*co-first author)
Hypertension. 2019 Oct;74(4):1041-1051. Download the full text article here.
Abstract - Transient hypertension is a risk factor for Alzheimer disease (AD), but the effects of this interaction on brain vasculature are understudied. Addressing vascular pathology is a promising avenue to potentiate the efficacy of treatments for AD. We used arterial spin labeling magnetic resonance imaging to longitudinally assess brain vascular function and immunohistopathology to examine cerebrovascular remodeling and amyloid load. Hypertension was induced for 1 month by administration of l-NG-nitroarginine-methyl-ester in TgF344-AD rats at the prodromal stage. Following hypertension, nontransgenic rats showed transient cerebrovascular changes, whereas TgF344-AD animals exhibited sustained alterations in cerebrovascular function. Human umbilical cord perivascular cells in combination with scyllo-inositol, an inhibitor of Aβ oligomerization, resulted in normalization of hippocampal vascular function and remodeling, in contrast to either treatment alone. Prodromal stage hypertension exacerbates latter AD pathology, and the combination of human umbilical cord perivascular cells with amyloid clearance promotes cerebrovascular functional recovery.
In vivo Neurovascular Response to Focused Photoactivation of Channelrhodopsin-2
James R Mester, Paolo Bazzigaluppi, Iliya Weisspapir, Adrienne Dorr, Tina L Beckett, Margaret M Koletar, John G Sled, & Bojana Stefanovic
NeuroImage. 2019 May 15;192:135-144. Download the full text article here.
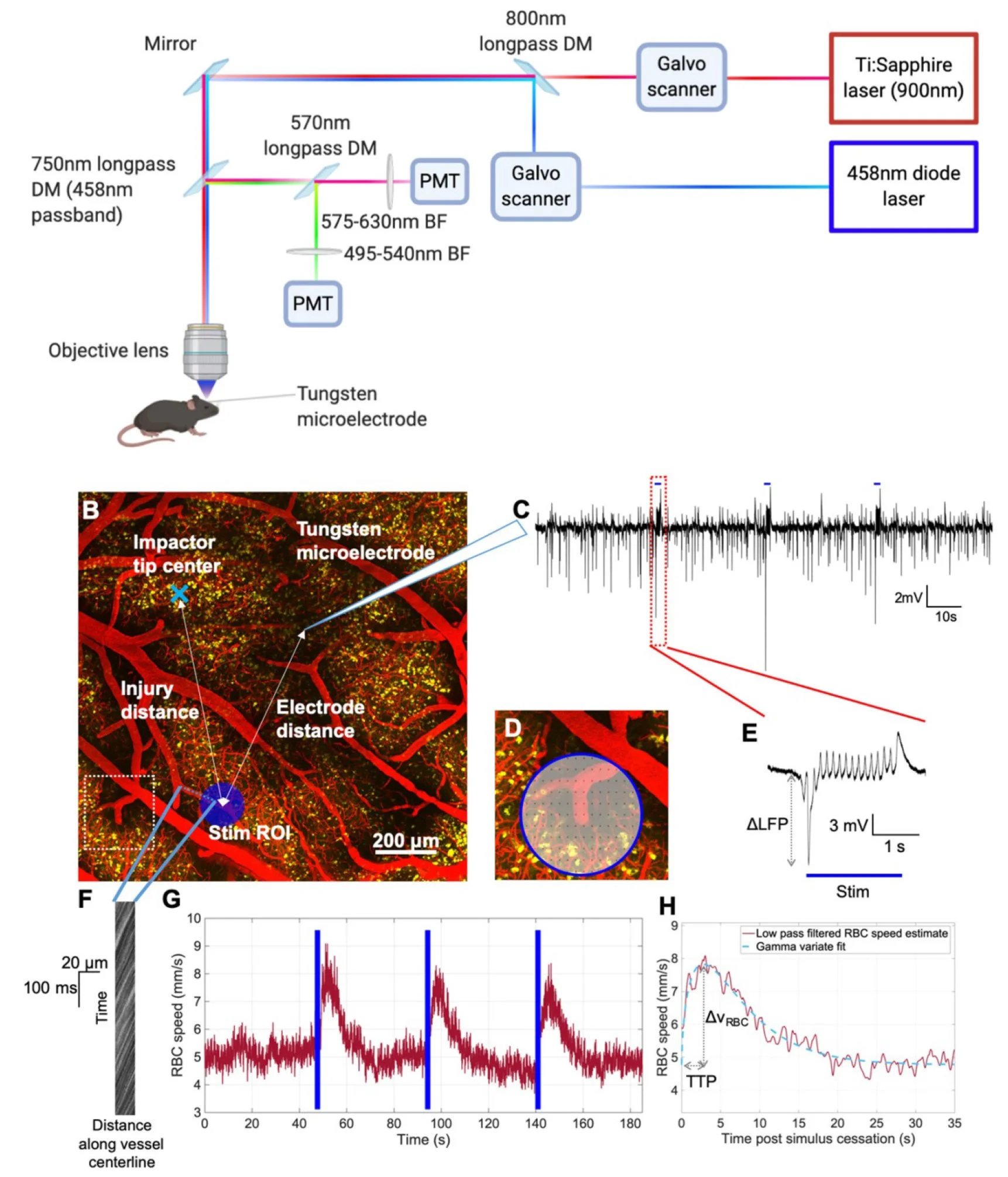
Abstract - The rapid growth in the use of optogenetics for neuroscience applications is largely driven by two important advantages: highly specific cellular targeting through genetic manipulations; and precise temporal control of neuronal activation via temporal modulation of the optical stimulation. The difference between the most commonly used stimulation modalities, namely diffused (i.e. synchronous) and focused (i.e. asynchronous) stimulation has not been described. Furthermore, full realization of optogenetics' potential is hindered by our incomplete understanding of the cellular and network level response to photoactivation. Here we address these gaps by examining the neuronal and cerebrovascular responses to focused and diffuse photostimulation of channelrhodopsin in the Thy1-ChR2 mouse. We presented the responses of photoactivation via 470-nm fiber optic illumination (diffuse) alongside 458-nm raster-scan (focused) stimulation of the barrel field. Local field potentials (LFP) assessment of intracerebral electrophysiology and two-photon fluorescence microscopy measurements of red blood cell (RBC) speed (vRBC) in cortical penetrating vessels revealed ∼40% larger LFP responses (p = 0.05) and twice as large cerebrovascular responses (p = 0.002) under focused vs. diffuse photostimulation (focused: 1.64 ± 0.84 mV LFP amplitude and 75 ± 48% increase in vRBC; diffuse: 1.14 ± 0.75 mV LFP amplitude and 35 ± 23% increase in vRBC). Compared to diffuse photostimulation, focused photostimulation resulted in a ∼65% increase in the yield of cerebrovascular responses (73 ± 10% for focused and 42 ± 29% for diffuse photostimulation) and a doubling of the signal-to-noise ratio of the cerebrovascular response (20.9 ± 14.7 for focused and 10.4 ± 1.4 for diffuse photostimulation). These data reveal important advantages of focused optogenetic photoactivation, which can be easily integrated into single- or two-photon fluorescence microscopy platforms, as a means of assessing neuronal excitability and cerebrovascular reactivity, thus paving the way for broader application of optogenetics in preclinical models of CNS diseases.
Refinement of a Chronic Cranial Window Implant in the Rat for Longitudinal in vivo Two-Photon Fluorescence Microscopy of Neurovascular Function
Margaret M Koletar, Adrienne Dorr, Mary E Brown, JoAnne McLaurin, & Bojana Stefanovic
Scientific Reports. 2019 Apr2; 9(1):5499. Download the full text article here.
Abstract - Longitudinal studies using two–photon fluorescence microscopy (TPFM) are critical for facilitating cellular scale imaging of brain morphology and function. Studies have been conducted in the mouse due to their relatively higher transparency and long term patency of a chronic cranial window. Increasing availability of transgenic rat models, and the range of established behavioural paradigms, necessitates development of a chronic preparation for the rat. However, surgical craniotomies in the rat present challenges due to craniotomy closure by wound healing and diminished image quality due to inflammation, restricting most rat TPFM experiments to acute preparations. Long-term patency is enabled by employing sterile surgical technique, minimization of trauma with precise tissue handling during surgery, judicious selection of the size and placement of the craniotomy, diligent monitoring of animal physiology and support throughout the surgery, and modification of the home cage for long-term preservation of cranial implants. Immunohistochemical analysis employing the glial fibrillary acidic protein (GFAP) and ionized calcium-binding adaptor molecule-1 (Iba-1) showed activation and recruitment of astrocytes and microglia/macrophages directly inferior to the cranial window at one week after surgery, with more diffuse response in deeper cortical layers at two weeks, and amelioration around four weeks post craniotomy. TPFM was conducted up to 14 weeks post craniotomy, reaching cortical depths of 400 µm to 600 µm at most time-points. The rate of signal decay with increasing depth and maximum cortical depth attained had greater variation between individual rats at a single time-point than within a rat across time.